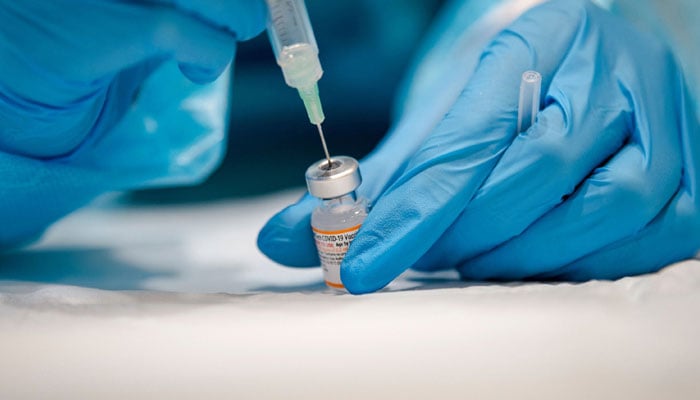
Fake vaccines given to pilgrims, reveals inquiry
Inquiry identifies that counterfeit vaccines were purchased from a noted pharmacy
ISLAMABAD: The federal capital’s drug control administration has uncovered a shocking scandal involving the administration of counterfeit vaccines to travellers and pilgrims at the official vaccination centre of the Federal Government Polyclinic (FGPC).
An inquiry, led by Sardar Shabbir Ahmed, Secretary of the Quality Control Board and Chief Drug Inspector of Islamabad Capital Territory (ICT), revealed that individuals seeking mandatory travel vaccines for the Kingdom of Saudi Arabia were being directed to purchase fake vaccines from a private medical store located outside the hospital.
A senior official from the Ministry of National Health Services, Regulations, and Coordination shared the inquiry’s findings with The News on Wednesday, saying that the malpractice involved systematic corruption by certain staff members of the FGPC vaccination centre.
The official explained that meningitis vaccines are mandatory for travellers to Saudi Arabia, as required by the Saudi Embassy. The FGPC vaccination centre issued prescription slips for the vaccine, which applicants were instructed to purchase independently before returning for administration. Detailed records, including applicants’ names, ages, genders, and vaccine types, were maintained in a register.
The inquiry identified that counterfeit vaccines were purchased from a noted pharmacy.
A search of the vaccination centre’s waste bin revealed several packs of used vaccine of noted brands, raising suspicions about the authenticity of the administered vaccines.
During their investigation, the team visited the pharmacy, where the owner admitted to selling five packs of a vaccine the previous day.
Alarmingly, two packs were obtained from an unauthorised peddler, and the pharmacy could not provide purchase documentation or warranties for the vaccines. Consequently, the pharmacy was sealed pending further investigation.
The authorised distributor for a pharmaceutical company confirmed that meningitis vaccine stocks had not been supplied since February 2024. Furthermore, the recovered Lot No. U6977QAB was never imported into Pakistan.
Photos of the recovered vaccine packs and their 2D barcodes were shared with the Drug Regulatory Authority of Pakistan (DRAP).
Verification through the Drug Regulatory Information System (DRIS) revealed no records of the identified lot number.
Additionally, inconsistencies in the expiration date were noted: the printed shelf life indicated November 2021, while the label showed November 2022.
The findings confirmed that the meningitis vaccine administered at FGPC was counterfeit and illegally sourced. This raises grave concern regarding public safety and vaccine administration practices, the official added.
The inquiry committee has recommended several measures to prevent similar incidents in the future, including the development of Standard Operating Procedures (SOPs) for vaccination centers. These SOPs should ensure accountability in vaccine administration, including the maintenance of detailed batch and lot number records for traceability.
Additionally, vaccination centres must provide applicants with information on precautions to take when purchasing vaccines.
Drug inspectors are urged to enhance surveillance to detect counterfeit vaccines and take strict action against violators.
The report also emphasised that DRAP must ensure the consistent availability of essential vaccines to curb the influx of counterfeit products.
-
 Columbia University Sacks Staff Over Epstein Partner's ‘backdoor’ Admission
Columbia University Sacks Staff Over Epstein Partner's ‘backdoor’ Admission -
 Ozzy Osbourne's Family Struggles Behind Closed Doors
Ozzy Osbourne's Family Struggles Behind Closed Doors -
 Dua Lipa Claims Long-distance Relationship 'never Stops Being Hard'
Dua Lipa Claims Long-distance Relationship 'never Stops Being Hard' -
 BTS Moments Of Taylor Swift's 'Opalite' Music Video Unvieled: See Photos
BTS Moments Of Taylor Swift's 'Opalite' Music Video Unvieled: See Photos -
 Robin Windsor's Death: Kate Beckinsale Says It Was Preventable Tragedy
Robin Windsor's Death: Kate Beckinsale Says It Was Preventable Tragedy -
 Rachel Zoe Shares Update On Her Divorce From Rodger Berman
Rachel Zoe Shares Update On Her Divorce From Rodger Berman -
 Kim Kardashian Officially Takes Major Step In Romance With New Boyfriend Lewis Hamilton
Kim Kardashian Officially Takes Major Step In Romance With New Boyfriend Lewis Hamilton -
 YouTube Tests Limiting ‘All’ Notifications For Inactive Channel Subscribers
YouTube Tests Limiting ‘All’ Notifications For Inactive Channel Subscribers -
 'Isolated And Humiliated' Andrew Sparks New Fears At Palace
'Isolated And Humiliated' Andrew Sparks New Fears At Palace -
 Google Tests Refreshed Live Updates UI Ahead Of Android 17
Google Tests Refreshed Live Updates UI Ahead Of Android 17 -
 Ohio Daycare Worker 'stole $150k In Payroll Scam', Nearly Bankrupting Nursery
Ohio Daycare Worker 'stole $150k In Payroll Scam', Nearly Bankrupting Nursery -
 Michelle Yeoh Gets Honest About 'struggle' Of Asian Representation In Hollywood
Michelle Yeoh Gets Honest About 'struggle' Of Asian Representation In Hollywood -
 Slovak Fugitive Caught At Milano-Cortina Olympics To Watch Hockey
Slovak Fugitive Caught At Milano-Cortina Olympics To Watch Hockey -
 King Charles Receives Exciting News About Reunion With Archie, Lilibet
King Charles Receives Exciting News About Reunion With Archie, Lilibet -
 Nvidia Expands AI Infrastructure With Nevada Data Centre Lease
Nvidia Expands AI Infrastructure With Nevada Data Centre Lease -
 Royal Family Shares Princess Anne's Photos From Winter Olympics 2026
Royal Family Shares Princess Anne's Photos From Winter Olympics 2026