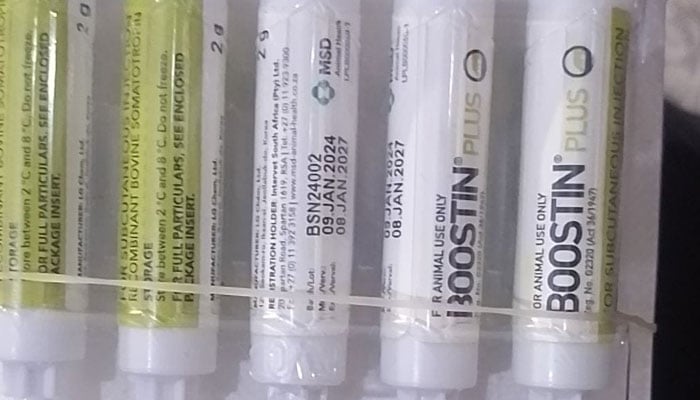
228 packs of Boostin injections seized in Malir
A joint operation by the Drug Regulatory Authority of Pakistan (Drap) and the Federal Investigation Agency (FIA) has led to the seizure of 228 packs of Boostin injections, an unregistered veterinary drug, in Karachi’s Malir area, officials said on Saturday.
According to Drap officials, the raid was carried out near the Anwar Baloch Hotel by a Drap team and FIA officials. The injections were seized, and Razak Khan, found in possession of the banned substance, was taken into custody, with an FIR registered against him.
The Boostin injection, containing the hormone bovine somatotropin (BST), was prohibited by Drap in 2015, after the authority’s registration board deemed it hazardous, and revoked its registration.
BST, commonly used to increase milk production in dairy cows by inhibiting cell death in mammary glands, has been banned in numerous countries due to its adverse effects on animal and human health.
Health risks associated with Boostin injections are numerous, as this hormone can cause significant harm to the animals, including reproductive and metabolic disorders.
On human health, residues from antimicrobial treatments for BST-related infections in cows can transfer to milk, potentially fostering antimicrobial resistance in humans, and increasing the risk of resistant bacterial infections, Drap officials said.
According to a Drap official, this raid marks the latest crackdown on the illegal circulation of BST-based products. “In June a Drap and FIA raid had unearthed a facility manufacturing Boostin injections in Karachi’s Clifton area, where large quantities of raw materials, finished products, and packaging machinery were seized.”
The official said that the unauthorised production, storage and sale of Boostin were found to be in violation of Section 18(1) of the Drugs Act of 1976.
It is worth mentioning here that the Supreme Court had reinforced the ban on BST injections by vacating a stay issued by the Sindh High Court, which had temporarily allowed the hormone’s use despite Drap’s cancellation order.
The apex court’s decision, supported by representatives from Drap and the Ministry of Health, reaffirms Pakistan’s commitment to public health, and aligns with bans in regions like the European Union, Canada and Australia.
The synthetic hormone, although registered by the US Food & Drug Administration in 1993, has been under scrutiny worldwide for its risks to human and animal health.
Experts, including Drap’s registration board officials, emphasise that BST can lead to cancers in the reproductive organs of cows, adversely affecting the quality of milk, and potentially leading to human health issues.
In response to the ongoing illegal supply, Drap has instructed its regulatory field force to step up market inspections to prevent the distribution of Boostin injections. Pharmacists and distributors are advised to immediately quarantine any stock of the product, and report their suppliers to authorities.
The public, especially dairy farmers, are cautioned against using BST-based products, and advised to consult veterinarians for any health issues in animals potentially related to the hormone.
-
 James Van Der Beek’s Family Faces Crisis After His Death
James Van Der Beek’s Family Faces Crisis After His Death -
 Courteney Cox Celebrates Jennifer Aniston’s 57th Birthday With ‘Friends’ Throwback
Courteney Cox Celebrates Jennifer Aniston’s 57th Birthday With ‘Friends’ Throwback -
 Camila Cabello Shares Update On Her Hair Two Years After Going Platinum
Camila Cabello Shares Update On Her Hair Two Years After Going Platinum -
 Prince William Steps In To Help Farmer's Awareness Mission
Prince William Steps In To Help Farmer's Awareness Mission -
 Queen Elizabeth Tied To Andrew's Sexual Abuse Case Settlement: Report
Queen Elizabeth Tied To Andrew's Sexual Abuse Case Settlement: Report -
 Mark Ruffalo Urges Fans To Boycott Top AI Company Boycott
Mark Ruffalo Urges Fans To Boycott Top AI Company Boycott -
 Prince William Joins Esports Battle In Saudi Arabia
Prince William Joins Esports Battle In Saudi Arabia -
 Princess Beatrice, Eugenie Are Being Ripped Apart: ‘Their Relationship Is Fully Fractured’
Princess Beatrice, Eugenie Are Being Ripped Apart: ‘Their Relationship Is Fully Fractured’ -
 Arden Cho Shares Update On Search For ‘perfect’ Wedding Dress Ahead Of Italy Ceremony
Arden Cho Shares Update On Search For ‘perfect’ Wedding Dress Ahead Of Italy Ceremony -
 Ariana Madix Goes Unfiltered About Dating Life
Ariana Madix Goes Unfiltered About Dating Life -
 Prince William Closes Saudi Arabia Visit With Rare Desert Shot
Prince William Closes Saudi Arabia Visit With Rare Desert Shot -
 Priyanka Chopra Breaks Silence On Rumors Questioning Marriage To Nick Jonas
Priyanka Chopra Breaks Silence On Rumors Questioning Marriage To Nick Jonas -
 'King Charles Acts Fast Or Face Existential Crisis' Over Andrew Scandal
'King Charles Acts Fast Or Face Existential Crisis' Over Andrew Scandal -
 Brooklyn Beckham Charging Nearly £300 In Ticket Cost For Burger Festival
Brooklyn Beckham Charging Nearly £300 In Ticket Cost For Burger Festival -
 Prince William Makes Unexpected Stop At Local Market In Saudi Arabia
Prince William Makes Unexpected Stop At Local Market In Saudi Arabia -
 Zayn Malik Shares Important Update About His Love Life
Zayn Malik Shares Important Update About His Love Life