Lack of local clinical data hindering treatment advancement: experts
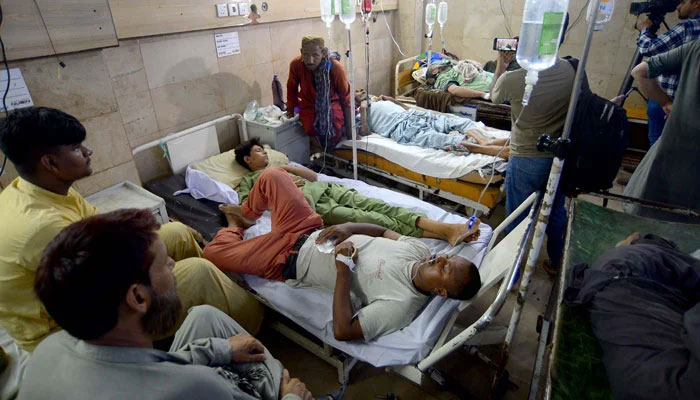
A lack of locally sourced data on infectious and non-communicable diseases is a major impediment to advancing health care in Pakistan, experts noted during a recent workshop on clinical trials. This absence of local data complicates efforts to develop treatments that address the specific genetic and health needs of Pakistan’s population.
To overcome this barrier, experts emphasised the need for more in-country clinical trials, and encouraged public participation in these efforts, similar to established practices in developed countries. Such contributions, they noted, are essential to achieve improved healthcare outcomes across Pakistan.
Organised as part of IQVIA Pakistan’s Corporate Social Responsibility initiative, the session titled ‘Understanding Clinical Trials and Key Stakeholders’ covered key topics, including the International Conference on Harmonisation’s Good Clinical Practice (GCP) guidelines, clinical research opportunities, and career pathways in clinical research.
Held in collaboration with the United Medical & Dental College (UMDC) and the Ziauddin University, the workshop aimed to provide students and faculty with a deeper understanding of the clinical research industry.
Syed Munawar Ali, head of clinical operations, highlighted Pakistan’s critical data gaps on diseases like hepatitis D and cardiovascular conditions. He pointed out that the absence of localised information hampers the development of effective, targeted therapies.
“Without a robust database and consistent local clinical trials, Pakistan remains reliant on foreign-developed treatments, which may not fully address our unique health needs,” he explained.
He called for stronger collaboration between academia and industry to bring Pakistan’s clinical research practices in line with global standards, particularly the GCP guidelines. He further urged young medical and science graduates to engage in research, and pursue ethical publications that represent Pakistan’s health statistics accurately.
Dr Zulfiqar Umrani, director of the Office of Research, Innovation and Commercialisation at ZU, reiterated the university’s commitment to participating in international clinical trials.
He noted the institution’s involvement in research on diseases such as rabies, Covid-19, and hepatitis C and D, emphasising the need for locally relevant data to ensure that treatments align with the needs of Pakistan’s population.
Dr Bilal Danish, director of the UMDC, stressed the importance of clinical trials as a core mission for his institution. He mentioned that the college actively involves both students and a dedicated research team in upcoming studies across Pakistan.
According to him, these research initiatives would enhance treatment options for local patients, and promote ethical practices in clinical research. He also highlighted the significance of these trials in establishing Pakistan as a key player in international clinical research, which he views as a critical step in showcasing the nation’s capabilities in healthcare innovation.
-
 Kate Middleton Proves She's True 'children's Princess' With THIS Move
Kate Middleton Proves She's True 'children's Princess' With THIS Move -
 Paul Anka Reveals How He Raised Son Ethan Differently From His Daughters
Paul Anka Reveals How He Raised Son Ethan Differently From His Daughters -
 'A Very Special Visitor' Meets Queen Camilla At Clarence House
'A Very Special Visitor' Meets Queen Camilla At Clarence House -
 Jodie Turner Smith Shares One Strict Rule She Follows As A Mom
Jodie Turner Smith Shares One Strict Rule She Follows As A Mom -
 Hailey Bieber Reveals KEY To Balancing Motherhood With Career
Hailey Bieber Reveals KEY To Balancing Motherhood With Career -
 Photo Of Jay-Z, Other Prominent Figures With Jeffrey Epstein Proven To Be Fake
Photo Of Jay-Z, Other Prominent Figures With Jeffrey Epstein Proven To Be Fake -
 Hillary Clinton's Munich Train Video Sparks Conspiracy Theories
Hillary Clinton's Munich Train Video Sparks Conspiracy Theories -
 Fans Slam Talk Show Host For 'cringe' Behavior In Chris Hemsworth Interview
Fans Slam Talk Show Host For 'cringe' Behavior In Chris Hemsworth Interview -
 Woman Jailed Over False 'crime In Space' Claim Against NASA Astronaut
Woman Jailed Over False 'crime In Space' Claim Against NASA Astronaut -
 James Van Der Beek’s Close Pal Reveals Family's Dire Need Of Donations
James Van Der Beek’s Close Pal Reveals Family's Dire Need Of Donations -
 Prince William And Harry's Cousins Attend 'Wuthering Heights' Event
Prince William And Harry's Cousins Attend 'Wuthering Heights' Event -
 Hailey Bieber Turns Heads Just Hours After Major Business Win
Hailey Bieber Turns Heads Just Hours After Major Business Win -
 King Charles' Andrew Decision Labelled 'long Overdue'
King Charles' Andrew Decision Labelled 'long Overdue' -
 Timothee Chalamet 'forever Indebted' To Fan Over Kind Gesture
Timothee Chalamet 'forever Indebted' To Fan Over Kind Gesture -
 Columbia University Sacks Staff Over Epstein Partner's ‘backdoor’ Admission
Columbia University Sacks Staff Over Epstein Partner's ‘backdoor’ Admission -
 Ozzy Osbourne's Family Struggles Behind Closed Doors
Ozzy Osbourne's Family Struggles Behind Closed Doors