US study finds mild side effects after Covid booster jabs
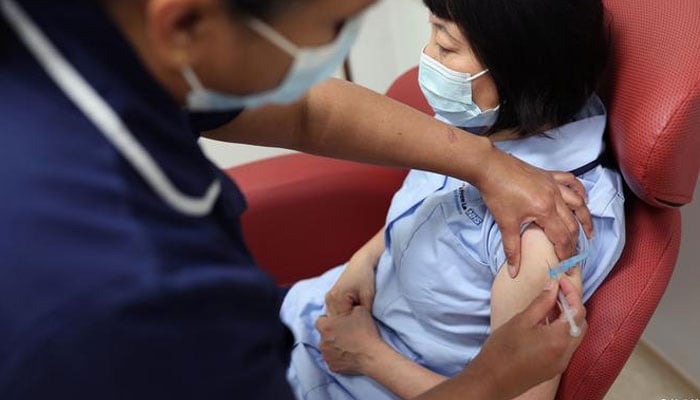
People who were inoculated Covid booster shots reported injection site pain (71 percent of study participants), fatigue (56 percent), and headache (43 percent).
WASHINGTON: Most side effects after a third dose of a Covid vaccine are mild or moderate, and occur at about as often as after shot two, a US study showed Tuesday in a finding that was expected but nonetheless reassuring.
The report by the Centers for Disease Control and Prevention (CDC) came from more than 22,000 people who signed up to a vaccine safety smartphone app and who received a booster shot between August 12 and September 19.
During this time, third doses were authorized for people who are immunocompromised, but not the wider population.
"The frequency and type of side effects were similar to those seen after the second vaccine doses, and were mostly mild or moderate and short lived," CDC director Rochelle Walensky said at a press briefing.
Frequently reported side effects included injection site pain (71 percent of study participants), fatigue (56 percent), and headache (43 percent).
Some 28 percent reported being unable to perform normal daily activities, usually the next day.
Medical care was sought by around two percent of participants, and 0.1 percent, that is 13 people, were hospitalized.
A subset of almost 21,700 who received the same mRNA vaccine (Moderna or Pfizer) for all three of their doses was further analyzed.
Among those who received Moderna, localized reactions -- such as arm pain -- were reported to be slightly more common after the third dose compared to the second.
Systemic reactions -- meaning those that occurred outside the injection site -- were slightly less frequent after the third dose compared to the second.
The same pattern occurred for Pfizer, and in both cases, the first shots resulted in significantly less frequent side effects, especially systemic, compared to shots two and three.
Last week, US health agencies expanded authorization of Pfizer booster doses to those aged over 65, those 18-64 with an underlying medical condition such as diabetes or obesity, and those who are especially exposed to the virus because of their work or where they live.
The CDC cautioned that there were certain limitations to its report.
These include the fact that signing up to the smartphone app called "v-safe" was voluntary and the participants skewed more heavily white than the national population.
During the study period, some non-immune compromised may have also received a booster outside of recommendations because they were worried about waning immunity, and so the findings can't reliably be linked to immune compromised people only.
-
All you need to know guide to rosacea
-
Prevent cancer with these simple lifestyle changes
-
Experts reveal keto diet as key to treating depression
-
Skipping breakfast? Here are some reasons why you shouldn't
-
Sciences reveals shocking body response against heart attack
-
Anti-inflammatory teas to keep your gut balanced
-
Emma Stone reveals she is ‘too afraid’ of her ‘own mental health’
-
5 simple rules to follow for smooth, healthy hair