Coronavirus vaccine over 90% effective, say Pfizer, BioNTech
"We are reaching this critical milestone in our vaccine development program at a time when the world needs it most," says Pfizer's chief executive Dr Bourla
Pfizer and BioNTech on Monday said that their jointly developed vaccine was more than 90% effective in preventing coronavirus, in what comes as a major development in the fight against the pandemic that has killed over a million people.
"Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19," said Dr Albert Bourla, Pfizer's chief executive in a statement.
According to initial findings, protection in patients was achieved seven days after the second of two doses, and 28 days after the first.
"The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine's ability to prevent COVID-19," Dr Bourla said.
"We are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis," he said.
"We are reaching this critical milestone in our vaccine development program at a time when the world needs it most."
Across much of the globe, coronavirus infections rates are hitting record highs, with hospital intensive care units filling up and death tolls mounting.
1.3 billion doses in 2021
Based on supply projections, the companies said they expect to supply up to 50 million vaccine doses globally in 2020, and up to 1.3 billion doses in 2021.
US biotech firm Moderna, several state-run Chinese labs, and a European project led by the University of Oxford and AstraZeneca are thought to be closing in on potentially viable vaccines.
Two Russian COVID-19 vaccines have been registered for us even before clinical trials were completed, but have not been widely accepted outside of Russia.
The Phase 3 clinical trial — the final stage — of the new vaccine, BNT162b2, began in late July and has enrolled 43,538 participants to date, 90% of whom have received a second dose of the vaccine candidate as of November 8.
Pfizer said it is gathering two months of safety data following the final dose — a requirement of the US Food and Drug Administration — to qualify for Emergency Use Authorization, which it expects by the third week in November.
"We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks," Bourla said.
Dozens more candidates
The so-called messenger RNA, or mRNA, vaccine is a new approach to protecting against viral infection.
Unlike traditional vaccines, which work by training the body to recognise and kill proteins produced by pathogens, mRNA tricks the patient's immune system to produce viral proteins itself.
The proteins are harmless, but sufficient to provoke a robust immune response.
The study also will evaluate the potential for the vaccine candidate to provide protection against COVID-19 in those who have had prior exposure to SARS-CoV-2, as well as vaccine prevention against severe COVID-19 disease.
Pfizer and BioNTech plan to submit data from the full Phase 3 trial for scientific peer-review publication.
As of mid-October, the World Health Organization (WHO) has identified 42 "candidate vaccines" at the stage of clinical trials, up from 11 in mid-June.
Ten of them were at the most advanced phase 3 stage, in which a vaccine's effectiveness is tested on a large scale, generally tens of thousands of people across several continents.
Stocks, oil prices rocket on coronavirus vaccine hope
European stock markets and oil prices rocketed after coronavirus vaccine hopes following the successful trials.
Already up strongly on Joe Biden's US election win, markets massively accelerated gains after Pfizer and BioNTech announcement.
London's benchmark FTSE 100 surged 5.5% in midday deals.
In the eurozone, Frankfurt jumped 6.0%, Paris soared 7.1%, Milan won 6.2% and Madrid 9.4%.
Dow Jones futures were up 5.4% ahead of the Wall Street open.
"Stock markets surged on some extremely positive news from Pfizer and Biontech," said Neil Wilson, chief market analyst at Markets.com.
"The Dow is now seen up 1,300 points (at the official open). Coming on top of the wave of relief from Joe Biden's victory, it's proving a spicy cocktail for stocks."
New York crude advanced 8.5% and Brent North Sea oil was up 7.8%.
The dollar was steady versus the euro and pound — and up more than one percent against the yen.
University of Oxford welcomes results
The University of Oxford welcomed the initial results and said that successful vaccines will offer the best possible results for humanity.
"Today's announcement indicates that it is possible to make a vaccine against this disease, which is fantastic news for everyone," it added.
'Such great news,' says Donald Trump
US President Donald Trump, reacting to the development, said that it was "such great news".
"STOCK MARKET UP BIG, VACCINE COMING SOON. REPORT 90% EFFECTIVE. SUCH GREAT NEWS!"
-
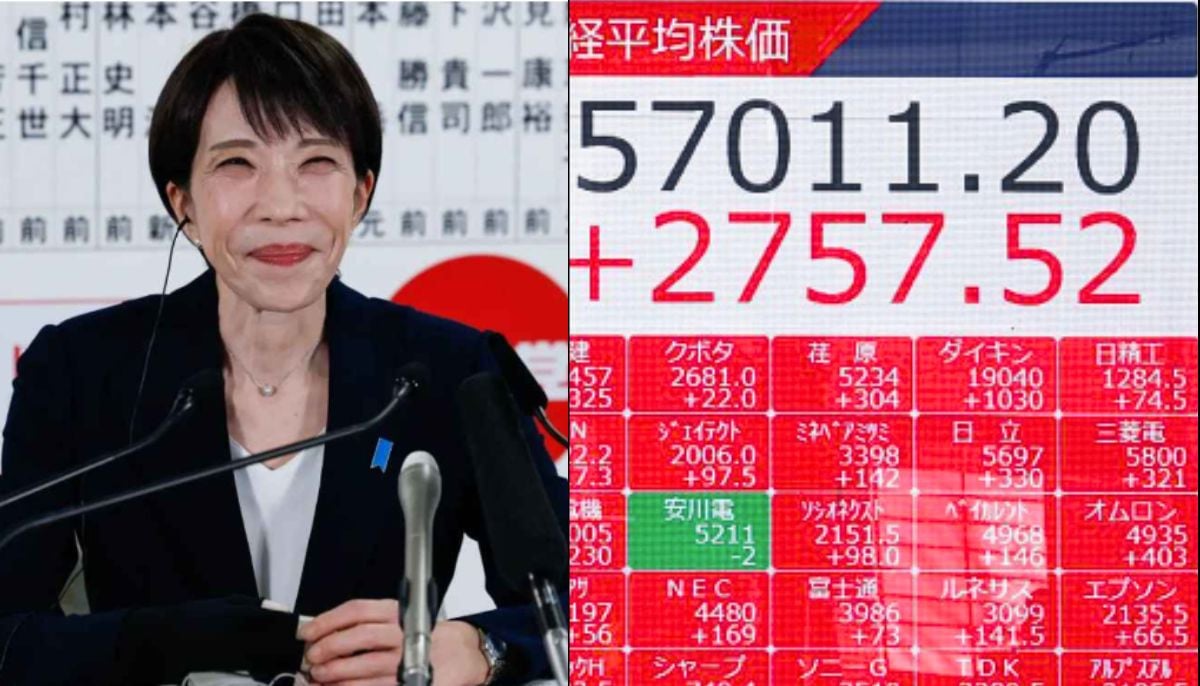
Japan Elections: Stock surges record high as PM Sanae Takaichi secures historic victory
-
$44B sent by mistake: South Korea demands tougher crypto regulations
-
South Korea: Two killed as military helicopter crashes during training
-
Jake Paul criticizes Bad Bunny's Super Bowl LX Halftime Show: 'Fake American'
-
Hong Kong court sentences media tycoon Jimmy Lai to 20-years: Full list of charges explained
-
Trump passes verdict on Bad Bunny’s Super Bowl halftime show
-
Blac Chyna reveals her new approach to love, healing after recent heartbreak
-
Melissa Jon Hart explains rare reason behind not revisting old roles